Solve[nt]ing The Mystery: A Case of Methanol Poisoning
…An 82-year-old male presents via EMS from home after he was found to be altered by family...
Toxicology cases can be some of the more challenging cases that present to the emergency department (ED). Often the patient is altered or there is minimal history available. Many cases involve the combination of more than one toxic ingestion or exposure which can lead to a complex clinical picture. The following case provides one such example where had the emergency department team not considered expanding their differential to include additional toxins, the patient may have had an adverse outcome.
case
An 82-year-old male with a past medical history including an abdominal aortic aneurysm, congestive heart failure, and coronary artery disease status post coronary artery bypass graft not on anticoagulation presents via EMS from home to the ED after he was found to be altered by family.
The family went to routinely check on the patient at his apartment approximately five hours prior to arrival to the ED. At that time, he was noted to be “disoriented and restless”. The back door was unlocked and the front door cracked open which family noted was unusual. Emergency medical services (EMS) were called at that time, but upon arrival the patient apparently seemed much improved and was ”more coherent”. He was thought to be at his baseline and thus he was not transferred to the ED. Family then encouraged the patient to take a nap.
When the patient woke up from his nap approximately an hour prior to arrival to ED, the patient’s mental status significantly worsened. The patient was uncharacteristically behaving aggressively toward family members and was drooling. Shortly after, he became lethargic. EMS was called yet again.
Upon EMS arrival to the scene the second time, the patient was speaking in full sentences but could not remember his family members' names. He appeared disheveled. Several pill bottles and an empty bottle of alcohol were scattered around the house. Per EMS, the patient’s initial blood pressure (BP) was 110/60 mmHg but dropped to 79/50 mmHg during transport. In the Trendelenburg position, his BP rose to 95/50 mmHg. Blood glucose was found to be mildly elevated. EMS reported he had left sided facial droop and right arm weakness. He had no known recent falls or trauma. The patient currently lived alone and was experiencing depression amidst a divorce with his wife.
The patient was noted to have the following history:
Social Hx:
Former tobacco smoker (cigarettes), 30 pack years (quit >30 yrs ago)
Occasional/social alcohol use
No other drug use known
Recent divorce from wife
Medications (found by EMS in wallet, consistent with pill bottles EMS found):
Amlodipine 5mg
Aspirin 81mg
Atorvastatin 40mg
Cilostazol 50mg
HCTZ 25mg
Levocetirizine 5mg
Lisinopril 20mg
Loratadine 10mg
Melatonin 3mg
Metoprolol Succinate 25mg
On arrival to the emergency department, he was activated as a code stroke.
The patient’s family was promptly called for additional collateral. They relayed that the patient had been depressed lately in the setting of a recent divorce. He had been sleeping more than usual. Family was unable to reach the patient over the phone for several days prior to finding him altered that day.
On exam:
Vital signs: Blood pressure 120/65 mmHg, heart rate of 107 beats per minute, temperature 98.1 degrees Fahrenheit, respiratory rate 16 breaths per minute, oxygen saturation 100% on room air
Pertinent exam: He was sleepy, but awakened to voice. There was no diaphoresis. His head was atraumatic and his pupils and equal and reactive. Pulmonary effort was normal. There were normal breath sounds bilaterally. His abdomen was soft and contender. He had normal range of motion throughout his upper and lower extremities. On neurological exam, the patient demonstrated poor concentration. He followed basic but not complex commands. He was oriented to person, place, and year but not to president. He had left facial asymmetry with smiling. He has dysarthria and slurred speech. He had 5/5 strength in all extremities. He was unable to ambulate.
A brain computed tomography was completely normal. Neurology did not think it was a stroke after evaluation. His electrocardiogram and chest x-ray were unremarkable.
The initial labs began to result. He was found to have an elevated BUN, creatinine, potassium and anion gap. His bicarbonate was found to be low. He was found to be acidotic on venous blood gas. He had an elevated serum osmolality. His urine was found to have many red blood cells and hyaline casts. He was negative for alcohol and Tylenol with an elevated salicylate level.
The ED team identified that the patient had a high anion gap metabolic acidosis which they believed to be in the setting of salicylate toxicity. The patient was given sodium bicarbonate and then was started on a bicarbonate drip. The ED team consulted nephrology who agreed with the plan for emergent dialysis in the intensive care unit (ICU).
Two hours later while the patient was still boarding in the emergency department awaiting a bed in the ICU and continued on a bicarb drip, labs were re-checked. His salicylate level was downtrending. However, his pH continued to trend downwards and he remained solemn. The team decided to send
That additional test that was sent was a methanol level (as well as ethanol and isopropyl alcohol levels).
discussion
This case has many critically important teaching points. Specifically, solving this case requires a good understanding of the differential diagnosis of high anion gap metabolic acidosis and understanding the utility of a serum osmolality level in certain cases. Moreover, equally important is the lesson to keep an open mind and not to anchor on one particular diagnosis, even if it may be contributing, as there may be multiple factors contributing to the patient’s presentation.
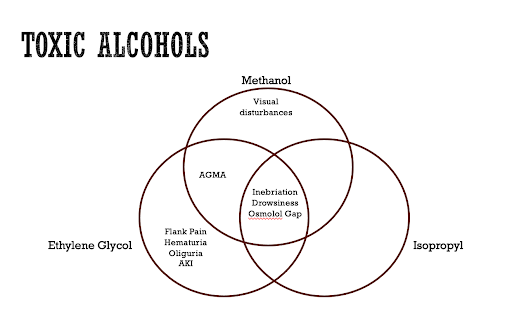
This discussion will focus primarily on methanol toxicity. However, given some degree in overlap of clinical presentation as well as similar risk factors for ingestion, it is worth mentioning two other toxic alcohols that can be seen in patients presenting with toxic ingestions to the emergency department, ethylene glycol and isopropyl alcohol.
Above is a Venn diagram comparing and contrasting the clinical presentations that can be seen with ingestion of each of these substances. Ingestion of each of these substances can result in inebriation, drowsiness, and lab findings of an osmolar gap. A key distinguishing feature between ethylene glycol and methanol compared to Isopropyl alcohol is the presence of an anion gap metabolic acidosis with the former two substances. Additional key distinguishing features include visual disturbances seen with methanol toxicity and flank pain, hematuria, oliguria, and acute kidney injury seen primarily with ethylene glycol toxicity. Importantly, as will be discussed below, a negative toxic alcohol level does not necessarily rule out the diagnosis completely. The remainder of this post will be primarily focused on the topic of methanol and its toxicity and how to manage it.
Methanol Overview
In current times, methanol is primarily used for the synthesis of other chemicals. It is commonly found in consumer products such as windshield washer fluid (which accounts for approximately 60% of ingestions seen in the United States), solid cooking fuel for camping, photocopying fluid, colognes and perfumes, and antifreeze. Ingestion of as little as one teaspoon can have serious toxic effects. Ingestion of 1g/kg is considered lethal if not treated.
History Clues for Methanol Toxicity
Obtaining collateral is often essential. This includes speaking with family, friends, and EMS. Asking about a history of alcohol use disorder or heavy drinking, depression, and suicide attempts is essential. It is also important to ask about what the patient does for work, and if they have had any exposure to automotive coolant/antifreeze, solvents, cleaners, fuels, or industrial products- either through work or hobbies. Ask about exposure to “wood alcohol” or “moonshine” as these are other names for methanol that the patient or collateral may be more familiar with. The patient may specifically complain of blurry vision, partial loss of vision, or other visual disturbances.
In reviewing the case presented above, this patient did have risk factors for methanol toxicity. In the initial history, it was noted by EMS that the patient looked “disheveled”, and that there were “several pill bottles and an empty bottle of alcohol” scattered around the house. Additionally, it was noted that the patient was currently experiencing depression in the setting of a recent divorce with his wife.
Clinical Presentations of Methanol Toxicity and some relevant Pathophysiology/Biochemistry
Inebriation: Inebriation is thought to be caused by activation of GABA-A receptors, similar to ethanol, and therefore presents similarly to ethanol intoxication with drowsiness, disinhibition, and slurred speech.
On the patient’s initial exam he was noted to be “sleepy, but awakened to voice”. He was also noted to have poor concentration as well as slurred speech, all of which are suggestive of inebriation.
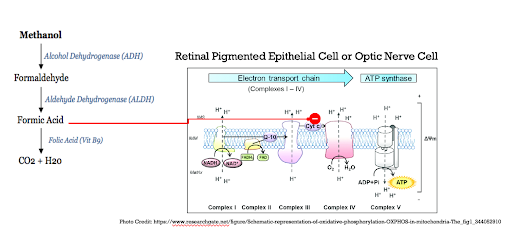
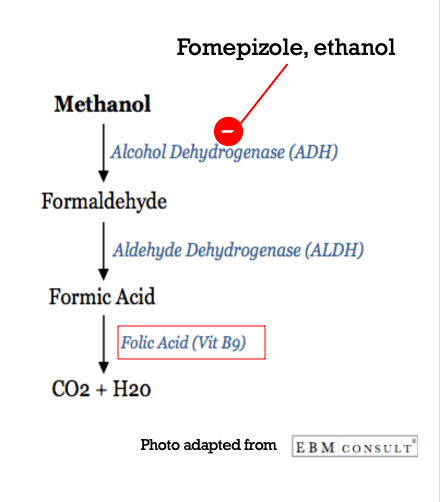
Anion Gap Metabolic Acidosis: Methanol initially contributes to the high anion gap metabolic acidosis when it is in its original form. It is metabolized by alcohol dehydrogenase (ADH) into formaldehyde. Formaldehyde is then metabolized by aldehyde dehydrogenase (ALDH) into formic acid. Formic acid is metabolized into carbon dioxide and water in a Folic Acid/Folate-dependent reaction.
Source: EBM Consult
On reviewing the patient’s labs in the above case, he presented with an anion gap metabolic acidosis. Further discussion on this below in the section dedicated to workup for methanol toxicity.
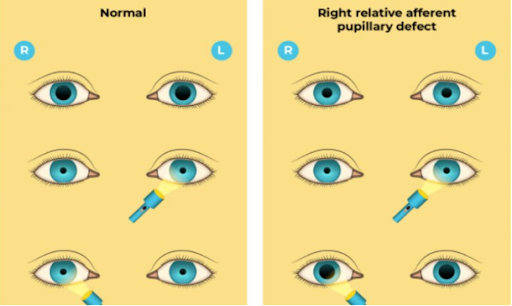
Other Neurologic and Ocular Manifestations: Symptoms may include blurry or hazy vision, color vision deficits, “snowfield vision”, or total blindness. Oddly, symptoms can often be asymmetric. Relevant exam findings that can be seen include a relative afferent pupillary defect when testing pupillary response, a central scotoma on visual field testing, and optic disc hyperemia, pallor, or papilledema on fundoscopic exam. It is very important to understand that this ocular damage is due to the toxicity of formic acid, the toxic metabolite of methanol, and not the parent compound itself.
Source: https://eyewiki.aao.org/Relative_Afferent_Pupillary_Defect#:~:text=6%20References-,Background,of%20the%20lateral%20geniculate%20body).
Source: https://www.webrn-maculardegeneration.com/central-scotoma.html
Source: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1442-9071.1992.tb00705.x
The mechanism by which methanol ingestion results in ocular toxicity is demonstrated in the below figure. Again, it is critically important to note that it is the formation of formic acid that ultimately results in visual deficits by the formic acid inhibiting cytochrome c of the electron transport chain in retinal pigmented epithelial and optic nerve cells. This results in impaired ATP production for energy use and ultimately damage to these cells that are critical for our vision.
On review of the patient case presented above, the patient may have subtly been showing signs of ocular toxicity. In the neurology resident exam, it was noted that he was not blinking to threat bilaterally, but was able to count fingers. This could represent findings of ocular toxicity.
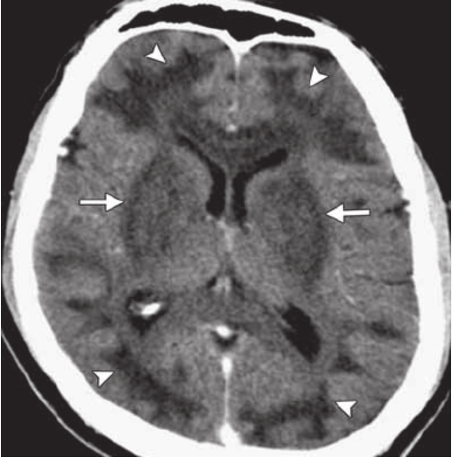
Basal Ganglia Abnormalities on Imaging: CT findings that can be seen with methanol toxicity in some cases include apparent ischemic or hemorrhagic abnormalities in the basal ganglia, such as the putamen in the case pictured below. These are typically bilateral. These are non-specific and are also seen in causes of prolonged hypoxia, prolonged hypotension, and carbon monoxide poisoning.
Source: Credit: https://www.researchgate.net/figure/Methanol-poisoning-in-a-41-year-old-man-who-presented-with-altered-mental-status-and_fig3_49777145
When should methanol toxicity be considered and what is the work up?
In addition to any history or physical exam findings that suggest possible methanol toxicity/ingestion, a helpful step in determining whether you should be concerned for acute methanol ingestion is to look at the bicarbonate level. Findings that are concerning for (but not specific for) methanol or other toxic alcohol ingestion include a patient with a very low bicarbonate level (often around 8mEq/L or less) and classically an elevated anion gap (though, depending on timing, not always present).
The next step would be sending methanol, ethylene glycol, and isopropyl alcohol levels (or a volatile alcohol panel). However, it is critical to understand that these levels will not be helpful to the clinician in the acute setting as they will not result fast enough to aid in the decision of whether to treat the patient for toxic alcohol toxicity. These tests are simply to confirm what is clinically suspected in the acute setting. For this reason, a very helpful (though still imperfect) test that will result much more quickly to help decide on treatment decisions is a serum osmolality level. This will be used to determine the osmolar gap.
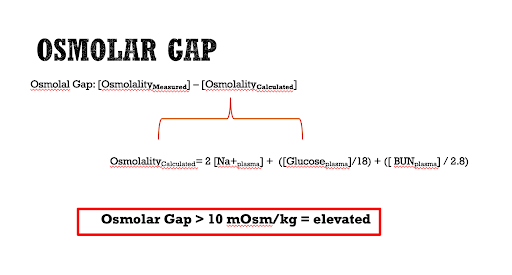
The Osmolar Gap
The osmolar gap is defined by the difference between the measured serum osmolality (the lab value of the serum osmolarity test that you sent to the lab) and the calculated serum osmolality. At baseline, the three main contributors to serum osmolality in the human body are sodium, glucose, and urea, and therefore these are used to calculate the expected serum osmolality, which is ultimately subtracted from the measured serum osmolality to determine the osmolar gap. A serum osmolar gap of greater than 10mOsm/kg is considered elevated. Aside from lab error, there are two main explanations for why the measured serum osmolality would be significantly higher than the calculated value.
The main explanation for a high serum osmolal gap is that there may be additional solute or solutes (aside from sodium, glucose, and urea) that are present in high enough concentrations to raise the measured serum osmolality. Examples of such solutes include ethanol, methanol, ethylene glycol, propylene glycol, isopropanol, mannitol, IVIG, sucrose, and maltose.
Once a serum osmolar gap has been found, the differential can be further broken down into solutes that typically have an associated high anion gap metabolic acidosis versus those that typically do not.
Methanol, ethylene glycol, and propylene glycol are the major causes of large osmolal gaps with associated high anion gap metabolic acidosis.
Finally, solutes that causes a high serum osmolal gap without an associated high anion gap metabolic acidosis include ethanol, isopropanol, diethyl ether, infusion of mannitol or sorbitol or glycine, and pseudohyponatremia such as in the setting of severe hyperlipidemia of hyperproteinemia, but this is outside the scope of this blog post.
In summary, if one has high clinical suspicion for methanol toxicity, sending a serum osmolality (as well as a methanol level or volatile alcohol panel) and then determining that the patient has a high serum osmolal gap with an associated high anion gap metabolic acidosis is generally an indication to treat for methanol toxicity empirically. However, important caveats are noted below.
Pitfalls in the Diagnostic Workup
A key concept to understand to help remember the pitfalls of the diagnostic workup for toxic alcohols is the following: it is the parent compound (i.e. methanol) that contributes to the osmolal gap, but it is the metabolite of the parents compound (i.e. formic acid) that produces the toxic effect on cells. In general, the earlier the patient presents to the emergency department after ingestion the higher the osmolal gap will be, the lower the anion gap will be, and the fewer symptoms there will be. The longer after ingestion they present, the serum osmolal gap may begin to decrease while the metabolic acidosis and toxic effects increase.
Diagnostic Pitfall #1: Assuming a low osmolal gap rules out the possibility of toxic alcohol ingestion.
A low serum osmolal gap does not rule out the possibility the patient ingested a toxic alcohol. For example, it may be that the patient had not been seen in days by family or friends and consumed a significant amount of methanol long before presentation. That patient may ultimately end up having a lower serum osmolality and osmolal gap on presentation to the ED because over time the methanol is metabolized to the toxic metabolite formic acid, which does not contribute to serum osmolality.
Diagnostic Pitfall #2: Being falsely reassured by an asymptomatic patient with a high serum osmolal gap and potential toxic alcohol ingestion.
A patient that you suspect or that you know ingested a toxic alcohol and has no symptoms and has a high serum osmolal gap is arguably the patient you should be the most worried about and the patient that you should be treating as soon as possible. The high serum osmolal gap without the patient being symptomatic may indicate that this was a very recent ingestion and that there are high levels of the parent compound present that have not yet been converted to the toxic metabolite. One must act quickly to prevent this.
ED Management of Methanol Toxicity (Basics)
As always, first address the ABCs (airway, breathing, circulation). Once these are addressed and you have high suspicion for methanol toxicity, the following steps should be taken (likely in addition to contacting the local poison control center):
Sodium Bicarbonate infusion: This stops the penetration of the toxic compound into cells. This is done by removing a hydrogen ion from formic acid to form the charged formate anion, which cannot readily cross the nonpolar/uncharged lipid cell membranes. See visual representation of this below.
Fomepizole (or, ethanol if fomepizole is unavailable): Fomepizole (and ethanol) inhibit alcohol dehydrogenase, the enzyme involved in the first step of metabolizing methanol into its toxic metabolite, formic acid.
Consult Nephrology for consideration of emergent hemodialysis
Administer cofactor/vitamin replacement (folic acid/B9, thiamine, pyridoxine): For example, folic acid is an important cofactor in the clearance of formic acid. See visual presentation below.
To summarize the case to this point, we have a patient known to be experiencing a depressive episode in the setting of a recent divorce presenting with altered mental status. EMS noted empty pill and alcohol bottles in the patient’s home. The patient appeared intoxicated with slurred speech on exam with possible visual abnormalities. His labs were notable for a very high anion gap metabolic acidosis with a very low bicarb level that could not be completely accounted for by other causes of acidosis and a mildly elevated salicylate level. He was additionally found to have an elevated serum osmolality. He was initially started on a bicarb drip for salicylate toxicity but his acidemia and mental status worsened despite a downtrending salicylate level.
Due to the significantly elevated osmolal gap and history concerning for possible toxic alcohol poisoning, he was then also treated empirically for toxic alcohol ingestion with ongoing sodium bicarbonate infusion, fomepizole, and vitamin replacement (including leucovorin and pyridoxine). He was admitted to the ICU where he underwent emergent dialysis for a total of three sessions. After this, his mental status quickly returned to baseline with normalization of his labs and he had no retinal toxic effects. On day 3 of his admission, he was transferred to the medical floor. On day 11 he was medically cleared and transferred to an inpatient psychiatry bed at a nearby facility for evaluation.
The patient’s methanol level ultimately resulted at 332 mg/dL.
take-aways
It is critical to obtain collateral from (ideally) multiple sources for a patient presenting with altered mental status. Each source may have additional details to offer.
Allow for the possibility that there may be multiple etiologies contributing to the patient’s presentation, particularly in the situation of toxic ingestion.
Methanol toxicity can present clinically as drowsiness, slurred speech, vision changes, a high anion gap metabolic acidosis, and elevated serum osmolality with a large serum osmolal gap.
Consider sending a serum osmolality level to calculate a serum osmolal gap as well a volatile alcohol panel (or methanol and ethylene glycol levels) in a patient with a high anion metabolic acidosis with a very low bicarbonate level (~8) that is not completely accounted for if the clinical context warrants.
Volatile alcohol panels (as well as individual tests for methanol, ethylene glycol, and isopropyl alcohol) do not result in adequate time to be used to make treatment decisions. Instead, the decision to treat for toxic alcohol ingestion should be based on clinical suspicion in combination with supportive lab findings such as a high osmolal gap (in the case of methanol and ethylene glycol) with a co-occuring AGMA.
Avoid pitfalls in diagnostic workup for toxic alcohol ingestions including assuming that a normal serum osmolal gap completely rules out toxic ingestion at any point or being falsely reassured that a patient with possible toxic alcohol ingestion with a high serum osmolal gap does not yet have symptoms of toxicity.
AUTHOR: Jake Gruber, MD is a recent graduate of Brown Emergency Medicine (2025).
FACULTY REVIEWER: Melanie Lippmann, MD is an Associate Professor at Brown Emergency Medicine.
references
Nelson, Lewis S. Goldfrank’s Toxicologic Emergencies. New York Mcgraw-Hill Medical, 2019.
UpToDate: Sections on “Methanol and ethylene glycol poisoning: Pharmacology, clinical manifestations, and diagnosis” and “Methanol and Ethylene Glycol Poisoning: Management”
“Methanol Toxicology Summary.” www.ebmconsult.com, www.ebmconsult.com/articles/methanol-toxicology#:~:text=Methanol%20toxicity%20is%20important%20to. Accessed 25 Sept. 2023.
“Figure 1. Schematic Representation of Oxidative Phosphorylation...” ResearchGate, www.researchgate.net/figure/Schematic-representation-of-oxidative-phosphorylation-OXPHOS-in-mitochondria-The_fig1_344052910.
“Relative Afferent Pupillary Defect - EyeWiki.” Eyewiki.aao.org, eyewiki.aao.org/Relative_Afferent_Pupillary_Defect#:~:text=6%20References-.
“Central Scotoma Causes, Symptoms and Help.” Www.webrn-Maculardegeneration.com, www.webrn-maculardegeneration.com/central-scotoma.html.
Stelmach, Maryla Z, and Justin O’Day. “Partly Reversible Visual Failure with Methanol Toxicity.” Australian and New Zealand Journal of Ophthalmology, vol. 20, no. 1, 1 Feb. 1992, pp. 57–64, https://doi.org/10.1111/j.1442-9071.1992.tb00705.x. Accessed 25 Sept. 2023.